Lannett Receives FDA Approval for Aspirin and Extended-Release Dipyridamole Capsules
On March 27, Lannett Company, Inc. (LCI) reported that they have received FDA approval for their Abbreviated New Drug Application (ANDA) for Aspirin and Extended-Release Dipyridamole Capsules, 25mg/200mg. The drug is the therapeutic equivalent to Aggrenox Capsules, 25 mg/200 mg by Boehringer Ingelheim Pharmaceuticals. Total annual sales in the US for Aspirin and Extended-Release Dipyridamole Capsules were $174.6 million.
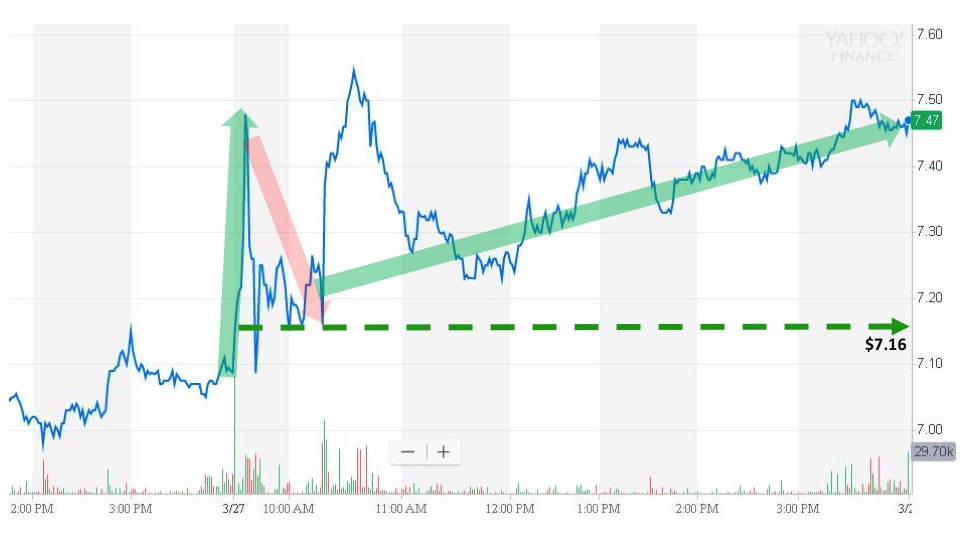
Rocket Tickers subscribers received an alert at 6:52 am. The next trade was for $7.20 at 7:14 am, and regular market trading opened for the day at $7.16. After an initial spike, the stock price faded during the first hour of regular market trading. Then, the price pushed higher throughout the remainder of the day to close at $7.47 with an event-day gain of 4.3%.
Visit the Knowledge Center for more information about clinical trials and how to trade them.
Check out our latest Live Webinar which provides more information about price patterns after clinical trial announcements and how to trade them.
Subscribe here if you would like to start receiving these signals in real-time and start trading!


Comments